Intelligent Technology: Impacting the Future of Clinical Research
May 5, 2021 | Today’s clinical trial landscape is incredibly complex to navigate. When compared to the entirety of the product lifecycle, clinical trials are rich with detailed processes used to create and validate the efficacy of a drug, at times driving disproportionately expensive and lengthy timelines. Among the myriad steps between start-up and completion, a prevailing issue is inefficiency—relying on years of approved and proven SOPs that can result in expensive delays and increased burdens for patients, sites and sponsors.
The good news is disruptive technologies—specifically artificial intelligence—are tangibly resolving challenges inherent to the clinical trial process. By harnessing intelligent, intuitive technology, teams are able to better orchestrate outcomes that help get much-needed treatments to patients faster with heightened productivity and efficiency.
Looking deeper into these technologies, there are several ways these innovations can help enhance efficiencies across clinical development.
Bringing Data Together
Today, a successful clinical trial both relies on and generates massive amounts of data, using myriad disparate technologies, collected mostly in siloed fashion. In fact, if you take all the data generated between the beginning of time and the year 2008, it is the same that is now generated every minute of every day.
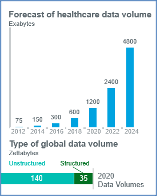
But it’s what we do with that data that makes it so valuable. Many sponsors struggle to bring disparate data together in a meaningful way for full visibility and transparency. This can be attributed to:
Outdated systems holding disparate data and failing to communicate with one another
Rapidly changing data making it nearly impossible to keep current and accessible to be used for trials
The rapid rise in data volume makes it unfathomable for humans to monitor and identify critical trends and provide deep insights
Also, because of the strict regulatory environment and industry mindset that has traditionally resisted change, it has been a difficult hurdle for life sciences to overcome.
By integrating the data and applying appropriate algorithms, life sciences companies can benefit from the deep analytics needed to make clinical trials more agile and adaptable, to ultimately increase productivity and efficiency to improve success rates.
Through AI analytics, sponsors can gain deep insights to better optimize many of the steps from start-up to close-out. This includes using AI with advanced and predictive analytics to improve adherence and safety and to predict and even prevent risk. Additionally, we can:
identify and propose optimal protocols
accelerate investigator and site selection
augment patient recruitment
improve patient monitoring
automate safety case processing
secure stronger endpoints
Increasing Efficiencies and Effectiveness
AI can be thought of as providing decision intelligence, working through data faster than humanly possible, connecting vital insights, and identifying trends hidden within. Advanced and predictive analytics highlight these trends and potential issues at both site and patient levels to enable faster, more informed decisions. It can literally be used to enhance the strengths that humans bring to the clinical trial process. AI can help automate repetitive tasks and reduce administrative burden to free staff for higher-level responsibilities, including spending more time with patients. This type of productivity could impact every phase of the clinical trial process and facilitate the rising use of virtual or decentralized trials.
It is important for sponsors and sites to remember that the goal here is to enable employees to do the right work at the right time to move healthcare forward. AI helps with workforce productivity, and as efficiencies are created, productivity is increased and the strengths of the workforce are augmented by the technology.
Improving the Potential for Success
The use of innovative technologies can help increase success rates. For example, some trials are employing synthetic arms, which have the potential to someday replace placebos. Others are doing in silico work, leveraging AI to determine whether or not a trial may be successful before it even starts.
Through technology, today’s portals, such as patient or site portals, support clear communication between the many stakeholders. And technology exists that integrates systems and data sources, so that AI algorithms can deliver thorough and accurate insights that can drive greater efficiencies and improved results.
The industry is at an inflection point with numerous decisions to be made. In order to transform clinical trials, life sciences companies need to consider use of disruptive technologies to better orchestrate outcomes in agile, adaptable trials for a future that promises improved patient care and outcomes at a time it matters most.
Nagaraja Srivatsan (“Sri”) is senior vice president and chief digital officer for R&DS Technology Solutions at IQVIA. He is responsible for driving growth and leading product development and operations of R&DS Technologies. Srivatsan has over 30 years of experience in growing businesses in the digital, data, AI, analytics, IT and operations management areas across industry verticals. He can be reached at nagaraja.srivatsan@iqvia.com.






