The Future of Clinical Trial Feasibility and Patient Outreach
By Tina Galasso, Bernadette Collins, and Paul Ivsin
May 5, 2015 | Contributed Commentary | Researchers and clinical study managers have been dreaming for years of the day when a patient’s every experience with the healthcare system is aggregated in a network of interoperable Electronic Medical Records (EMRs) that can be seamlessly cross-referenced. When that day comes, assessing the feasibility of clinical trials and even delivering information on clinical trials to potential patients will be easier than ever before. While we will not enjoy this ideal anytime soon, there is currently the potential to use a combination of data sources—both EMRs as they exist today and other patient-level databases—to support feasibility assessment and patient outreach for clinical research.
The Current State of EMRs
The U.S. Department of Health and Human Services (HHS) has published a strategic plan to guide widespread adoption of health IT from 2015-2020. The Plan’s goals are to increase the electronic collection and sharing of health information to improve health, healthcare, and reduce costs. Among the more specific objectives imbedded in these broad goals is one to advance research, scientific knowledge, and innovation.
Although HHS is developing a “National Interoperability Roadmap,” EMR systems are not yet capable of sharing “patient health information electronically across organizational, vendor, and geographic boundaries.” This lack of interoperability, of course, limits the current application of electronic records for clinical research purposes. However, there are innovative approaches being developed now to serve as a workable stopgap until patients’ complete medical histories are captured in a universal database.
Feasibility Assessment Using EMR and Prescription/Medical Claims Data
Even today, EMR data (derived from system vendors and large provider networks), can be combined with longitudinal prescription data and medical claims data to estimate the number of eligible patients for a given clinical trial. The combination of data sources is important because, at this point, none contains all of the necessary medical details on patients to assess their suitability for a trial. For example, EMR data include vital statics and lab values that may not be available in medical claims data.
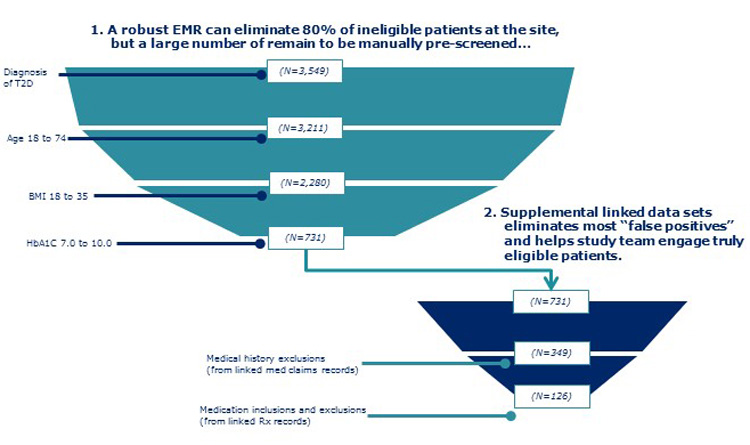
In this methodology, depending on the trial protocol, various data assets are used alone or in combination to estimate patient counts. The starting point is to search local site EMR databases for potentially eligible trial participants. This initial list can then be cross-referenced against prescription and/or medical claims databases to assess each patient’s eligibility according to trial inclusion/exclusion (I/E) criteria that cannot be determined from the EMR record. As can be seen in the “attrition funnels” presented in Figure 1, no dataset on its own is sufficient, but collectively, they are able to address the full set of I/E criteria, to produce a realistic estimate of eligible patient counts.
Patient Outreach at Site of Care, Pharmacies
In the foreseeable future, it should be possible for clinicians to receive electronic alerts of trials of potential interest to specific patients during the treatment visit and based on a comprehensive medical history.
A modified form of such alerts is possible today, albeit based only on the information contained in the host EMR system. For example, a Principal Investigator (PI) working at a large university health system may, after entering a patient’s data, see an automated message about the patient’s possible suitability for a given trial. That alert, however, will likely have many false positives, because it will be based only on the contacts the patient had with the health system; it will be missing information about the patient’s visits with other, external physicians and facilities.
With the right database linkages, researchers would be able to link individual EMR systems, prescription records, and medical claims databases automatically to develop a more complete patient profile against which the trials’ I/E would be matched. The alerts that PIs see would, therefore, be much more refined and targeted.
Similarly, large pharmacy systems can, and do, identify patients who may be suitable for clinical trials and contact them on behalf of trial sponsors. However, once again, the search is generally limited by the scope of the information in the pharmacy system. With links to EMR systems data and claims data, pharmacy service providers would be able refine their matches of patients to potential trial candidates using a more comprehensive set of I/E. This ability to better target patients should dramatically increase the success rate and return on investment of such outreach programs.
Clearly, creating the type of database linkages and automatic searches just discussed will require coordination across entities, careful contracting between parties, and ensuring that the methodologies conform to all privacy legislation. However, the necessary technology, information, and market demand exist today to make these concepts a reality.
All three authors work in Clinical Trial Optimization Solutions at IMS Health. Tina Galasso is Senior Manager, Data Services team; Bernadette Collins is Senior Manager, Data Services team; and Paul Ivsin is Director, Offerings Development.






